COVID-19 integrated surveillance system
What is it?
The integrated microbiological and epidemiological surveillance for COVID-19 continuously and systematically collects, compares and analyzes information on all cases of SARS-CoV-2 infection confirmed by molecular diagnostics at regional reference laboratories across Italy. It is a necessary and useful tool for informing the general population on the impact and evolution of the epidemic and helping health authorities make public health decisions.
When was it established?
Circular n. 1997 of 22 January 2020, issued by the Ministry of Health, established the COVID-19 surveillance in Italy, setting out the first criteria and methods for reporting cases of SARS-CoV-2 infection (at that time still known as “novel coronavirus”) jointly agreed with the Department of Infectious Diseases of the Istituto Superiore di Sanità (ISS) [1]; as the epidemiological situation evolved, further ministerial circulars containing additional information and updates were issued [2]. By Order n. 640 of 27 February 2020, the Civil Protection entrusted the epidemiological and microbiological surveillance for COVID-19 to the ISS [3].
Coordination
The COVID-19 surveillance system is coordinated by the Department of Infectious Diseases of the ISS. Each Region/Autonomous Province has identified one or more local coordinators.
How are the data collected?
Data on laboratory-confirmed SARS-CoV-2 infections are provided on a daily basis to the ISS by all Regions/Autonomous Provinces. The ISS has created a dedicated IT platform which allows data to be either collected through a web interface linked to the platform or sent directly as datasets. The Department of Infectious Diseases of the ISS processes and analyzes the data on the platform and makes them available to ensure monitoring of the epidemic across the country.
- View the key national data of the COVID-19 integrated surveillance system.
Molecular diagnostics procedures
Respiratory samples are the specimens of choice for diagnosing SARS-CoV-2 infection. Generally, an upper respiratory specimen is collected via nasopharyngeal and oropharyngeal swabs [3]. According to the U.S. Centers for Disease Control and Prevention (CDC), anterior nares and nasal mid-turbinate swabs can also be used [4]. Occasionally a lower respiratory specimen, such as an endotracheal aspirate (ETA) or bronchoalveolar lavage (BAL) sample, can be collected, where available.
The biological sample is transported to the laboratory at a controlled temperature. RNA from the sample is then extracted and purified to allow possible detection of the viral RNA using a rapid molecular testing method known as Reverse Real-Time PCR (rRT-PCR).[5]
Is the integrated surveillance system designed to include all laboratory-confirmed COVID-19 cases?
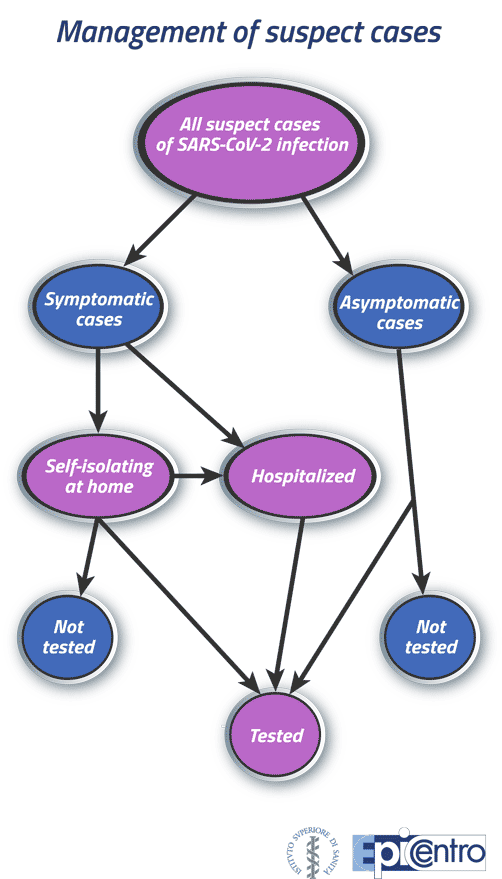
The surveillance system is designed to include all laboratory-confirmed COVID-19 cases (Figure 1). Symptomatic/paucisymptomatic clinical cases and symptomatic at-risk contacts are prioritized for diagnostic testing [6]. Therefore, some asymptomatic cases may not be tested.
Figure 1: Management of suspect cases
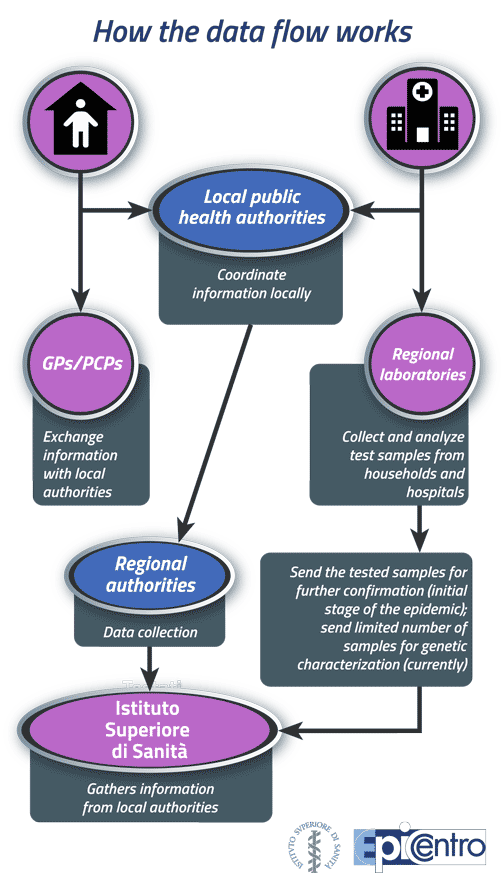
COVID-19 positive cases: how the data flow works
The information used in the national COVID-19 surveillance system comes from a complex data flow starting at the local level. Suspect COVID-19 cases, whether self-isolating at home or hospitalized, undergo SARS-CoV-2 molecular diagnostic testing. Samples are sent to the regional reference laboratories to be analyzed. The results are reported to the Local Health Units (LHUs), which coordinate the data flow between cases, hospitals, General Practitioners (GPs) and Primary Care Paediatricians (PCPs) to gather detailed information on each positive case. The regional coordinators of the COVID-19 epidemiological surveillance will then collect information about the cases from all LHUs.
At the initial stage of the epidemic, the regional laboratories would send all SARS-CoV-2 positive samples to the ISS for the diagnosis to be confirmed; currently, they are sending only a limited number of samples for genetic characterization (See Figure 2).
Figure 2: Data flow in the surveillance system for COVID-19 positive cases
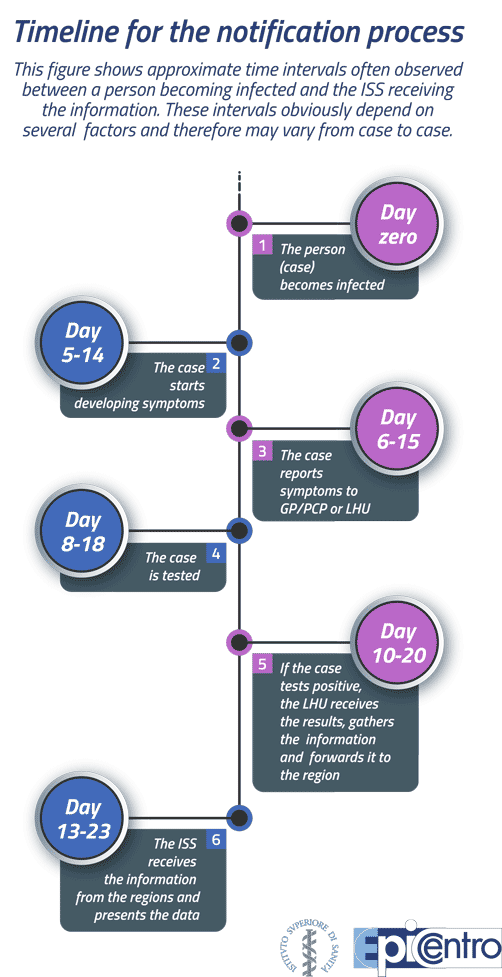
Timeline for the notification process
The time lag between the moment a person becomes infected with SARS-CoV-2 and the moment the notification of the disease is recorded in the COVID-19 integrated surveillance system varies considerably, but is on average 2-3 weeks. This depends on a number of factors relating to the characteristics of the infection and the infected person, as well as the organization of local and/or regional health services. In almost all cases, it takes 5 to 14 days for an individual to develop symptoms of the disease (incubation period), and a diagnostic swab test is often carried out only after symptom onset. If the infected person is a close contact of a positive case, they may be tested before developing any symptom, but are unlikely to be diagnosed as positive until after 4-5 days from infection, because the number of virus particles replicating during the first few days is not large enough for them to be detected by current diagnostic tests. If the person has not already been identified as a close contact and does not suspect that they may be infected, they might wait a few days after symptom onset before contacting their GP or visiting an emergency department; based on the symptoms, their severity and an assessment of the possible risk of exposure to SARS-CoV-2, the doctor may wait a few more days before referring the patient for a swab test. The time needed for the test to be performed and for the laboratory to provide a diagnosis will depend on the number of test referrals made in the patient’s area, the available staff and the capacity of the laboratory. Finally, after a positive laboratory result is obtained, there may be a variable delay before the relevant information is sent to the ISS based on the organizational and management procedures in place in the region. Figure 3 is a schematic representation of the timeline for the notification process, showing approximate time intervals from “day zero”, i.e. when the infection occurs.
Figure 3: Timeline for the notification process
Difference (delay) between the information provided by the Civil Protection and that provided by the ISS
In Italy, the two main sources of information about the COVID-19 epidemic are the Civil Protection (CP) and the ISS. The data they provide may differ, as both their nature and the collection methods are different.
Every day, the Civil Protection collects information on the total number of positive tests, deaths, hospitalizations and intensive care admissions in all Italian provinces. By contrast, the ISS requests that Regions provide individual-level details on all cases, including demographic data, clinical conditions and comorbidities. Aggregated data have the advantage of being faster and easier to collect compared to individual-level data, but the latter allow more detailed and accurate analyses.
It should be noted that the COVID-19 integrated surveillance may be subject to changes and/or adjustments as a result of the gradual easing of lockdown restrictions from 4 May 2020 and the constantly evolving epidemiological, health and legislative situation.
- Download the document “COVID-19 integrated health surveillance privacy information” (in Italian, pdf 477 kb).
- Ministerial Circular n. 1997 of 22 January 2020 (in Italian, pdf 476 kb)
- Ministry of Health. Novel coronavirus. Regulations, Circulars and Orders (in Italian)
- Order of the Head of the Civil Protection n. 640 of 27 February 2020 (in Italian)
- CDC guidelines: "Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19 (updated 5 May 2020)"
- WHO document “WHO Interim guidance Laboratory testing for coronavirus disease (COVID-19) in suspected human cases” (Updated 19 March 2020)
- Ministerial Circular n. 11715 of 3 April 2020 (in Italian)
